The phenomenon known as the photoelectric effect stands as a testament to the astonishing and sometimes perplexing behavior of light. It was first observed by Heinrich Hertz in 1887. Hertz’s experiments laid the foundation for the subsequent work on the photoelectric effect by other scientists especially it was Einstein who, in 1905, provided the groundbreaking explanation of the photoelectric effect, demonstrating that light behaves not only as a wave but also as a stream of particles, known as photons. They noticed that whenever a metal surface at a higher threshold frequency is exposed to electromagnetic radiation, then radiation can be absorbed & the electrons are emitted. In this article, we are going to discuss the phenomenon of the photoelectric effect which uses a material that absorbs electromagnetic radiation & produces electrically charged particles. Beyond its theoretical implications, the photoelectric effect has found practical applications, revolutionising fields such as technology, astronomy, and materials science.
What is the Photoelectric Effect?
The photoelectric effect is one type of phenomenon that happens whenever a light incident on a metal surface then it causes the electrons to eject from that metal. It was noticed that only certain light frequencies are able to cause the electrons ejection which is known as photoelectrons. If the incident light’s frequency was very low then electrons were not ejected, even if the light intensity was too high. If the light frequency was higher, then electrons were ejected from the surface of the metal even if the light intensity was too low. This smallest frequency required to cause electron ejection is known as the threshold frequency.
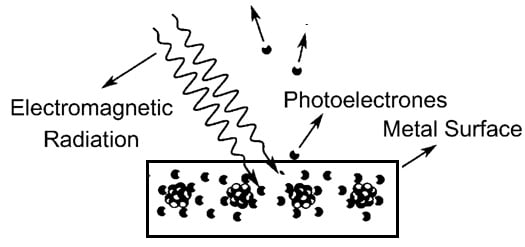
Photoelectric Effect
The photoelectric effect mainly depends on different factors like; light frequency, intensity, material’s nature, potential difference, and light energy. There are many factors that affect the photoelectric effect are; the incident radiation’s intensity, the potential distinction between the metal plate & collector, and the incident radiation’s frequency.
This photoelectric phenomenon can be simply explained by the nature of light particles, where light can be visualized as a flow of electromagnetic energy particles. These light particles are known as photons. The light energy held by a photon is related to the light frequency through Planck’s equation E = h𝜈 = hc/λ
Where,
‘E’ is the photon’s energy.
‘h’ is Planck’s constant
‘𝜈’ is the light frequency.
‘c’ is the light’s speed.
‘λ’ is the light’s wavelength.
Therefore, it can be understood that different frequencies of light carry photons of varying energies. For example, the frequency of blue light is greater than that of red light (the wavelength of blue light is much shorter than the wavelength of red light). Therefore, the energy held by a photon of blue light will be greater than the energy held by a photon of red light.
Photoelectric Effect Equation Derivation
The photoelectric effect was explained by Einstein based on Planck’s quantum theory which states that light radiation moves in the discrete photons form. The energy carried by every particle of light mainly depends on the frequency of light is shown below.
E = hν
Where ‘h’is Planck’s constant (6.6261 × 10-34 Js) and ‘v’ is the light’s frequency.
We know that light is bundled up into photons so whenever a photon drops on the metal surface then the energy of the entire photon can be transmitted to the electron. Emitted electrons from the metal surface can lose some kinetic energy throughout the collision. But the surface electrons carry all the kinetic energy imparted by the photon and have the maximum kinetic energy. The photoelectric effect formula is given below.
The energy of photon = Required energy to eject an electron + Maximum electron’s kinetic energy.
E = W + KE where E = hν.
hv = W + KE.
KE = hv – w.
The ‘ν0’ electrons at the threshold frequency are just ejected & do not have any K.E (kinetic energy). Under this frequency, there is no emission of electrons. So, the photon energy with this frequency should be the metal’s work function.
w = hv0
So, the Maximum K.E equation will become KE = 1/2mv2max = hv–hv0
1/2mv2max = h(v−v0)
Here, ‘Vmax’ is the maximum K.E of the electron. Experimentally it can be calculated through the stopping potential. So, stopping potential (ev0) = 1/2mv2max. So, Einstein explained simply the Photoelectric effect with the light’s particle nature.
Photoelectric Effect Circuit Diagram
The photoelectric circuit is shown below which is very helpful in studying the photoelectric effect characteristics. This circuit has an evacuated glass tube, A – metal plate & B – -photosensitive plate. Here ‘B’ plate acts as an emitter and the ‘A’ plate acts as a collector. To study the photoelectric effect characteristics, we have to study the light intensity effect, the potential & the frequency above the photocurrent.
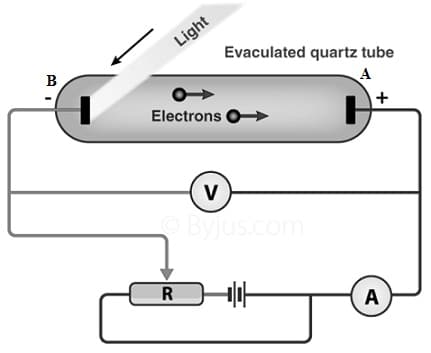
Photoelectric Effect Circuit
To study the intensity of the effect, whenever plate ‘A’ is maintained at maximum potential as compared to plate B, the incident light’s intensity can be changed with other parameters being fixed, after that it is noticed that the photocurrent linearly changes with the light intensity.
To study the potential effect, whenever the plate-A is maintained at a +ve potential, it is noticed that first, the photocurrent enhances, after that, it turns into maximum & gets saturation value. So the photocurrent saturation value is known as saturation current. Alternatively whenever a negative potential is provided, then the photocurrent will drop gradually to zero for a fixed negative potential value which is called the stopping potential.
To study the frequency effect, whenever the different lights with different frequencies and the same intensities are incident over the glass tube, it is noticed that the stopping potential is negative mainly for higher frequencies. Therefore, we have studied simply all the photoelectric effect characteristics with the above setup.
Characteristics
The characteristics of the photoelectric effect include the following.
- The threshold frequency will change by the material because it is not the same for different materials.
- The stopping potential is proportional directly to the frequency & the process is instant.
- The photoelectron’s kinetic energy (K.E) is directly proportional to the frequency of light.
- The photoelectric current is proportional directly to the intensity of light.
Advantages
The advantages of the photoelectric effect include the following.
- The photoelectric effect is significant because it offered the former experimental evidence that light can act as both a wave & a particle which is known as wave-particle duality.
- This effect is the foundation for many modern technologies like solar cells and photocells.
- The photoelectric effect-based devices have numerous attractive properties; like generating a current that is proportional directly to the intensity of light & a very quick response time.
- The photoelectric effect study led to significant steps in knowing the quantum light nature.
Limitations
The limitations of the photoelectric effect are discussed below.
- The photoelectric effect is pretty limited to working with only some elements.
- The range of light is short within the wavelength spectrum that ranges from 400 nm to 700 nm.
- This effect can be explained only by the element nature of light only but not wave.
- The problems with the effect are; the nonexistence of a lag time, the independence of the K.E of photoelectrons on the incident radiation’s intensity & the existence of a cut-off frequency.
Applications
The applications of the photoelectric effect include the following.
- This effect is used in Imaging technology that has image intensifiers and television camera tubes.
- This effect is used to study the nuclear phenomenon.
- It is used to analyze the materials chemically based on their electron emission.
- This effect is used to derive theoretical information regarding different energy conditions of electrons within atoms throughout the transition
- This effect is used in solar cells & solar energy production.
- This is used in motion sensors & position sensors.
- This is used in lighting sensors in smartphones, which allow automatic screen brightness adjustment based on the lighting because the amount of generated current through this effect depends on the light-hitting intensity of the sensor.
- This is used in Digital cameras for detecting as well as recording light because they use photoelectric sensors that react to different light colors.
- This is used in XPS or X-Ray Photoelectron Spectroscopy.
- This is used for detecting low light levels within the photomultiplier.
- This is used in Night vision devices.
What is Photoelectric Emission used for?
The photoelectric emission is used for generating electricity using solar panels. This panel includes metal that helps in generating electricity by simply producing energy whenever the light drops on the metal.
Why Does Photoelectric Emission Occur?
The photoelectric emission mainly occurs whenever the incident light is above a certain minimum frequency known as threshold frequency.
Why Photoelectric Effect Cannot be Explained?
This effect cannot be explained by wave theory due to many reasons: Electrons are emitted after some time whenever light drops a material based on wave theory. But, the electron emissions within the photoelectric effect immediately occur without delay.
What are the Two Laws of Photoelectric Effect?
The laws of this effect are; that the electron emission from the surface of metal will stop after a certain frequency called as the threshold frequency. The emitted electron from the metal surface is directly proportional to the incident light’s intensity.
Why is the Photoelectric Effect Important?
The photoelectric effect plays a key role in solar cells because this effect changes light energy to electrical so that solar energy can be changed easily to electricity. There are some more applications of this effect like light networking, photocopy machines, pollution monitoring devices & detection sensors.
Does the Photoelectric Effect Depend on Intensity?
The photoelectric effect depends on light intensity because when the light intensity is enhanced the photoelectric effect will reduce. When the intensity of light reduces the photoelectric effect will increase.
Thus, this is an overview of the photoelectric effect that occurs whenever the photons within the light interact through the electrons within the metal and every photon will interact with a single electron. The incident photon energy is helpful in emitting the electrons from the metal surface & to pass on energy toward the emitted electrons. To emit electrons, the minimum required energy from the metal surface is known as the work function. So the incident photon’s energy must be higher as compared to the work function. Here is a question for you, what is an atom?