Dielectric material acts as an insulating medium between two conducting bodies. It has the properties of insulators but shows the effect of the electric field. All-dielectric materials can be called as insulators, but all insulators are not dielectric materials. Because of its insulating properties, dielectrics are used widely in the field of electrical and electronics engineering. This article discusses an overview of dielectric materials, working, types, examples, properties, and applications.
What is Dielectric Material?
Definition: Dielectric is the materials that do not allow electricity to flow within them but show electric effects. They can be also defined as a substance that does not allow the flow of charges through it permits them to exert electric field and force. The dielectric material supports the electric field but opposes the flow of charges. It is regarded as an insulating medium. In the process of supporting the electrostatic field, it dissipates energy in the form of heat.
Formation of Electric Field-Polarization.
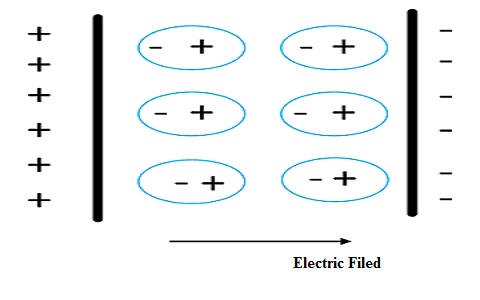
Dielectic Material
As shown in the above figure, the two thick lines represent two conducting medium, just like plates of a capacitor. The circles inside represent the molecules of the dielectric. When the plates are applied potential difference, as shown by positive and negative symbols, the charges inside dielectric align themselves accordingly. The opposite charges of the molecules dielectric cancel themselves and remaining charges which align towards the conducting plates, form an electric field as indicated by the arrow in the figure. That’s why it is said that dielectric material opposes the conduction of charges but forms the electrostatic field. It also forms the electric force due to the field. This is defined as polarization.
Dielectric Examples
Different examples of dielectric exist in all three forms of matter. Examples of solid dielectrics are mica, porcelain, glass, plastic, etc. Dielectric in the form of liquid includes transformer oil, distilled water, mineral oil, etc. Dielectric in the form of gas includes air, hydrogen, nitrogen, Sulphur, helium, etc. The absence of air i.e. vacuum also is one best dielectric material used. Due to its molecular properties, a vacuum is considered as one of the efficient dielectric material.
A dielectric opposes the flow of charges and at the same time dissipates energy in the form of heat which is known as dielectric loss. Based on the loss, the dielectric material is characterized. The less heat loss, the more effective is the material. One more parameter, which characterizes dielectric material is dielectric constant. Less is the constant, better is dielectric. Materials with less dielectric constant include, perfect vacuum, dry air, gasses such as hydrogen and helium, etc.
The next category of dielectrics include dielectrics with moderate dielectric constant. These include ceramics, distilled water, paper, glass, polyethylene, etc. Also, metals with high oxides have a moderate dielectric constant.
Types of Dielectric Material
There are two types of material
- Polar Dielectric Material
- Nonpolar dielectric Material
Polar Dielectric Material
The term polar indicates, there is a net polar movement in the absence of an electric field. This means that in the absence of an electric field there is the distance between positive and negative .charges. They have internal dipole movement in the absence of an electric field. For example, if we consider the molecular structure of H20, we have two atoms of hydrogen and one atom of oxygen. The two atoms of hydrogen, there is a distance of separation between the center of mass for positive charge and negative charge.
The center of mass for positive charges is formed by hydrogen atoms. Similarly, the center of mass for negative charges is formed by oxygen atoms. There is a separation between these two centers of charges, which causes dipole movement. There will be a dipole movement from the two centers of charges, even in the absence of an electric field. Similarly, HCl and NaCl are examples of polar dielectric materials.
Hence the polar molecule can be defined as a molecule in which the center of mass of positive charge does not coincide with the center of mass of negative charge in the absence of electric field. A substance made up of a polar molecule is defined as a polar dielectric. One more point to be noted is in polar molecules, the size of molecules is not the same. For example in H20, the size of the hydrogen molecule is small as compared to the size of the oxygen molecule.
Nonpolar Dielectric Material
In non-polar dielectrics, the size of all molecules is the same. For example CO2, the size of carbon and oxygen molecule are the same. This causes, the center of mass of positive charge and center of mass of negative charge to coincide with each other. This means that there is no distance of separation between the two masses. This causes the net dipole movement to be zero. Hence in a non-polar dielectric, it can be defined as a molecule in which the center of mass of positive charge coincides with the center of mass of negative charge in the absence of electric field. A substance made of up non-polar molecule forms the non-dielectric. Examples are CO2, H2 N2, etc. The net dipole movement of non-polar dielectric material is zero.
Properties of Dielectric Constant
The properties of dielectric materials are listed below:
- Low Dielectric Constant- Dielectric materials must have a low dielectric constant.
- Low Dielectric loss- The dielectric loss of the material must be less. This is calculated in the form of heat dissipation. It is also called a low loss factor
- High Dielectric Strength- The dielectric strength of the material must be high
- Temperature Stability- Its performance must be stable under high-temperature conditions. Like porcelain which has excellent temperature stability
- Storage Stability – it must have the property to store energy.
- Good Frequency Response – Since elements like capacitors are used under high-frequency conditions, so dielectric materials must have a good frequency response.
Applications of Dielectrics
Due to its properties, dielectric materials have a number of applications. A few of them have been enlisted below-
- Dielectrics materials are used for making capacitors. Based on the dielectric material, the capacity of the capacitor is analyzed. The dielectric material causes a separation between the plates.
- It is used for manufacturing insulating materials which are used in overhead transmission lines. Like porcelain. In this application, the properties of dielectrics are very important, since they are used at very high voltage.
- Transformer Oil- In transformers, oil is a very vital component since it is used for cooling and insulation.
- Mica- Mica or paper dielectric is also used for the insulating of winding in transformers.
- They are used in manufacturing semiconductors since based upon the dielectric constant, the performance of semiconductors improves.
- They are used for manufacturing piezoelectric, ferroelectric, etc. widely.
- The dielectric material is also used for display applications such as LCD etc.
FAQs
1). Which material is used as a dielectric?
Ans. A common material used for dielectric is mica, paper, transformer oil, porcelain, etc.
2). What is the use of dielectric?
Ans. Dielectric forms insulation between two conducting mediums
3). Why dielectric material is used in a capacitor?
Ans. Dielectric is used in a capacitor because it forms the separation between two conduction plates and enables the capacitor to store energy in the electric field. Due to the properties of the dielectric, the capacitor can store energy in the electric field.
4). Is water a dielectric?
Ans. Yes, water is dielectric. It is made up of polar molecules, hence it can be categorized as the polar dielectric.
5). What is a perfect dielectric?
Ans. An ideal dielectric is a material which exhibits infinite insulation, or zero conductivity, and has only displacement current. A perfect dielectric is equivalent to a capacitor that can store energy.
know more about Electrostatic Precipitator.
Hence we have seen the properties of dielectric and dielectric materials. Based on the properties and dielectric constant value, dielectric has numerous applications. Mostly used for manufacturing capacitors, other common applications are insulators in the overhead transmission line. On increasing the potential across the dielectric material causes dielectric breakdown, also called as flashover. Formation of dust, poor maintenance, aging, etc. are other common problems for dielectric failure. Here is a question for you, how does dielectric play a role in increasing the capacity of the capacitor?